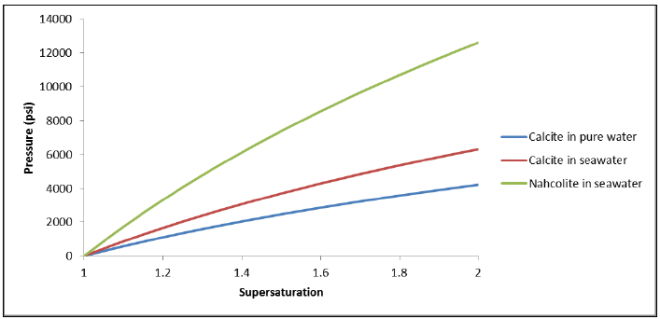
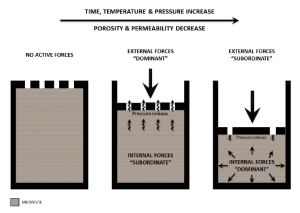
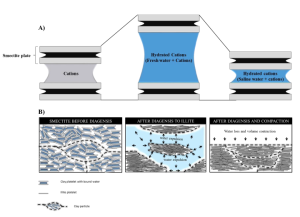
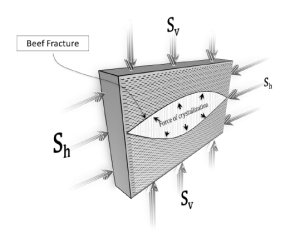
Flow Rate Conversion:
According to,
\[ 1 m^3/s = 543440 bbl/day
\]
\[ 1 cm^3/hr = (543440)(10^{-6})/3600 bbl/day
\]
Thus,
\[ 1 bbl/day = (3600)(10^6)(543440) = 6624.46 cm^3/hr
\]
Alternatively,
\[ 1 bbl = 0.158987 m^3
\]
Thus,
\[ 1 bbl/day = (0.158987)(10^6)/(24) = 6624.46 cm^3/hr
\]
If the fluid is water with density of $1 g/cm^3$,
\[ 1 bbl/day = 6624.46 g/hr
\]
Multiply specific gravity, SG, to covert to other fluids.
Darcy Equation Radial Flow:
\[ q_{well}=-0.006328\frac{2\pi kh\left(p_{e}-p_{well}\right)}{B\mu\ln\left(r_{1}/r_{w}\right)}=-0.039765\frac{kh\left(p_{e}-p_{well}\right)}{B\mu\ln\left(r_{1}/r_{w}\right)}
\]
where, the conversion factor,
\[\frac{[mD][psi]}{[cp]}= \frac{0.9869233 \times 10^{-15} \times 10.7639[ft^2][psi]}{10^{-3} \times 0.000145038 [psi] \frac{1}{60\times 60\times 24}[day]} = 0.006328 \frac{[ft^{2}]}{[day]}
\]
\[ 0.006328\frac{[mD][ft][psi]}{[cp]}=\frac{[ft^{3}]}{[day]}
\]
If using $bbl/day$ instead of $ft^3/day$, $1\,bbl=5.6146\,ft^{3}$.
\[ \frac{0.006328\times2\pi}{5.146}=0.001127\times2\pi=0.007082=\frac{1}{141.2}
\]
thus,
\[ q_{well}=-\frac{kh\left(p_{e}-p_{well}\right)}{141.2B\mu\ln\left(r_{1}/r_{w}\right)}
\]
However, for gas flow, the bottomhole flow rate has to be converted to standard conditions ($T_{sc}=60 F = 520 R$ and $P_{sc} = 14.7 psi$).
\[ q_{g}=\frac{p_{sc}}{T_{sc}}\frac{zT}{p}q_{g,sc}
\]
Substitute in horizontal radial flow equation (steady-state, homogeneous):
\[ \frac{p_{sc}}{T_{sc}}\frac{zT}{p}q_{g,sc}=\frac{0.006328\times\left(2\pi rh\right)k}{\mu}\frac{dp}{dr}
\]
Integrate from wellbore to reservoir boundary:
\[ \frac{Tq_{g,sc}}{kh}\ln\left(\frac{r_{e}}{r_{w}}\right)=0.703\int_{p_{wf}}^{p}\frac{2p}{\mu z}dp
\]
Assume viscosity and z-factor are constant and ensure the unit of $q_{g,sc}$ is in $Mscf/day$ (thousand standard cubic feet per day):
\[ q_{g,sc}=\frac{kh\left(p_{ave}^{2}-p_{wf}^{2}\right)}{1424T\mu z\ln\left(\frac{r_{e}}{r_{w}}\right)}
\]
Diffusivity Equation:
Unit conversion factors of 0.006328 and 0.007082:
According to Darcy's law:
\[ q=0.001127\frac{kA\triangle p}{\mu L}
\]
where, $q$ is in $bbl/day$; $k$ is in $mD$; $\triangle p$ is in $psi$; $\mu$ is in $cp$; $L$ is in $ft$.
Due to the conversion of 1 $bbl$ = 5.6146 $ft^3/day$,
\[ q=(0.001127)(5.6146)\frac{kA\triangle p}{\mu L}=0.006328\frac{kA\triangle p}{\mu L}
\]
For radial flow of incompressible fluids:
\[ v=\frac{q}{A}=0.001127\frac{k}{\mu}\frac{dp}{dr}
\]
where, $A=2\pi rh$. Thus, by integration,
\[ \int_{r_{1}}^{r_{2}}\frac{q}{2\pi rh}dr=0.001127\int_{p_{1}}^{p_{2}}\frac{k}{\mu}dp
\]
Hence,
\[ q=(2\pi)(0.001127)\frac{kh\triangle p}{\mu\ln(r_{2}/r_{1})}=0.007082\frac{kh\triangle p}{\mu\ln(r_{2}/r_{1})}
\]
where, $q$ is in $bbl/day$; $k$ is in $mD$; $\triangle p$ is in $psi$; $\mu$ is in $cp$; $L$ is in $ft$.
Gas Diffusion and Adsorption:
Diffusion Coefficient:
\[ D^{k*}=10^3\frac{b^K k^\infty}{\mu}
\]
where, $D^{k*}$ in $cm^2/s$, $b^K$ in atm, k in mD, $\mu$ in cp = $mPa \cdot s$, so
\[ cm^2/s=10^3\frac{atm \cdot mD}{cp}=10^3\frac{10^5Pa \cdot 10^{-12} m^2}{10^{-3}Pa\cdot s}=10^{-4}m^2/s
\]
Multiply by $(93)$ convert from $cm^2/s$ to $ft^2/day$.
Gas Diffusion:
Solute Concentration:
Molality:
Also called molal concentration, is a measure of the concentration of a
solute in a solution in terms of amount of substance in a specified amount of mass of the
solvent.
A commonly used unit is
mol/kg. A solution of concentration 1
mol/kg is also sometimes denoted as 1
molal.
The molality ($b$), of a solution is defined as the amount of substance (in $mol$) of solute, $n_{solute}$, divided by the mass (in $kg$) of the solvent, $m_{solvent}$:
\[ b = \frac{n_{solute}}{m_{solvent}}
\]
Molarity:
Molar concentration, also called molarity, amount concentration or substance concentration, is a measure of the concentration of a solute in a solution, or of any chemical species in terms of amount of substance in a given volume. A commonly used unit for molar concentration used in chemistry is
mol/L. A solution of concentration 1
mol/L is also denoted as 1
molar (1 M).
Molar concentration or molarity is most commonly expressed in units of moles of
solute per litre of
solution. For use in broader applications, it is defined as amount of solute per unit volume of solution, or per unit volume available to the species, represented by lowercase $c$:
\[ c = \frac{n}{V} = \frac{N}{N_{\rm A}\,V} = \frac{C}{N_{\rm A}}
\]
Here, $n$ is the amount of the solute in moles, $N$ is the number of molecules present in the volume $V$ (in litres), the ratio $N/V$ is the number concentration $C$, and $N_A$ is the Avogadro constant, approximately $6.022×10^{23} mol^{−1}$.
Or more simply: 1 molar = 1 M = 1 mole/litre.
PPM (parts-per-million):
Parts-per notation is often used describing
dilute solutions in chemistry. When working with aqueous solutions, it is common to assume that the density of water is 1.00 $g/mL$. Therefore, it is common to equate 1 gram of water with 1 mL of water. Consequently, $ppm$ corresponds to 1 $mg/L$ and $ppb$ corresponds to 1 $μg/L$.
Conversions:
The conversions to and from the molar concentration, $c$, for one-solute solutions are
\[ c = \frac{\rho\, b}{1+ b M},\ b=\frac{c}{\rho-cM}
\]
where $\rho$ is the mass density of the solution, $b$ is the molality, and $M$ is the molar mass of the solute.
To be exact, ppm is interchangeable with molality solute through molecular weight,
\[ 1 ppm = \frac{10^{3} mol/kg}{M_w}
\]
Because it is usually easier to measure liquids by volume instead of mass, molarity (M) is defined as the number of moles of solute ($n$) divided by the volume ($V$) of the solution in liters. Thus,
\[ 1 ppm = \frac{10^{3} mol/L}{M_w}
\]
This is only valid for dilute solution. As salt concentration increases, the volume of solution varies (larger or smaller) and the deviation enlarges.
Friction Pressure:
Friction Head Loss:
\[
h_{f}=\frac{2f_{f}V^{2}L}{g_{c}d}
\]
where, $f_{f}$ is Fanning Friction Factor, quarter of Moody Factor.
Friction Pressure:
\begin{eqnarray*}
p_{f} & = & \frac{2f_{f}V^{2}\rho L}{d}=\frac{2f_{f}(q/A)^{2}\rho L}{d}=\frac{2f_{f}(\frac{4q}{\pi d^{2}})^{2}\rho L}{d}=2\frac{16}{\pi^{2}}\frac{f_{f}q^{2}\rho L}{d^{5}}\\
& = & 2\frac{16}{\pi^{2}}\frac{\left[bpm\right]^{2}\left[lbm/ft^{3}\right]\left[ft\right]}{[in]^{5}}=2\frac{16}{\pi^{2}}\frac{\left[\frac{5.6146ft^{3}}{60s}\right]^{2}\left[\frac{lbm}{ft^{3}}\right]\left[ft\right]}{[in]^{5}}\\
& = & 2\frac{16}{\pi^{2}}\frac{\left(\frac{5.6146}{60}\right)^{2}\left[ft^{3}\right]\left[lbm\frac{ft}{s^{2}}\right]}{[in]^{5}}=2\frac{16}{\pi^{2}}\left(\frac{5.6146}{60}\right)^{2}\frac{\left(12\right)^{3}\left[lbm\frac{ft}{s^{2}}\right]}{in^{2}}\\
& = & 2\frac{16}{\pi^{2}}\left(\frac{5.6146}{60}\right)^{2}\left(12\right)^{3}\frac{1}{32.174}\frac{lbf}{in^{2}}\\
& = & 1.52484psi
\end{eqnarray*}