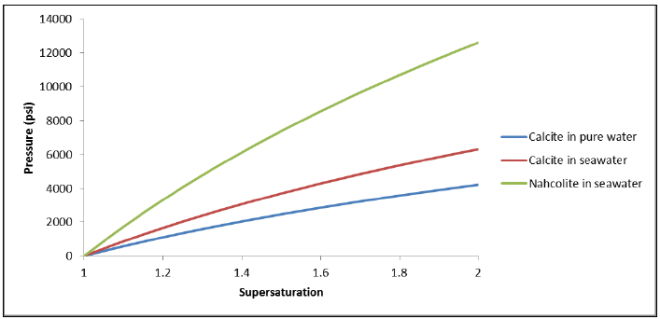
Equation of State:
Ideal gas: $pV = RT$
Real gas: Peng-Robinson EOS
\[ p=\frac{RT}{(V-b)}-\frac{\alpha a}{V^{2}+2Vb-b^{2}}
\]
$ a = \frac{0.457235\,R^2\,T_c^2}{p_c} $
$ b = \frac{0.077796\,R\,T_c}{p_c} $
$ \alpha = \left(1 + \kappa \left(1-T_r^{\,0.5}\right)\right)^2 $
$ \kappa = 0.37464 + 1.54226\,\omega - 0.26992\,\omega^2 $
$ T_r = \frac{T}{T_c} $
In terms of compressibility, $Z=PV/(RT)$, and in polynomial form:
$ A = \frac{a\alpha p}{ R^2\,T^2} $
$ B = \frac{b p}{RT} $
$ Z^3 - (1-B)\ Z^2 + (A-2B-3B^2)\ Z -(AB-B^2-B^3) = 0 $
where $\omega$ is the acentric factor of the species, R is the universal gas constant. Possible roots, 1 three real roots (largest, gas volume; smallest liquid volume); 2 one real (true volume) two imaginary.
The fugacity of pure component is given by,
\[ \ln\phi=\ln\frac{f}{p}=Z-1-\ln(Z-B)-\frac{A}{2\sqrt{2}B}\ln\left(\frac{Z+2.414B}{Z-0.414B}\right)
\]
Mixing rules:
Parameters of PR equation for the mixture of N simple fluids where molar percent of the i-th component is zi are defined by mixing rules
\[ a=\sum_{i}\sum_{j}z_{i}z_{j}a_{ij}
\]
\[ b=\sum_{i}z_{i}b_{i}
\]
where, $a_{ij}=(1-\delta_{ij})\sqrt{a_{i}a_{j}}$
Here aij is expressed through empirically determined binary interaction coefficient δij characterizing the binary formed by component i and component j.
Some δij are tabulated (e.g. table 4.2 in Pedersen and Christensen(2006)). For those one which are not tabulated formula of Chueh and Prausnitz (1967) could be used:
\[ 1-\delta_{ij}=\sqrt{\frac{2V_{c,i}^{1/6}V_{c,j}^{1/6}}{V_{c,i}^{1/3}V_{c,j}^{1/3}}}
\]
Formula for fugacity coefficient of the mixture, calculated via PR equation and standard mixing rules, has expression (see Peng and Robinson (1976) or equation 4.65 in Pedersen and Christensen (2006) for more detail)
\[ \ln\phi_{i}=\ln\frac{f_{i}}{z_{i}p}=\frac{b_{i}}{b}(Z-1)-\ln(Z-B)-\frac{A}{2\sqrt{2}B}\left(\frac{2}{a}\sum_{j}^{N}z_{j}a_{ij}-\frac{b_{i}}{b}\right)\ln\left(\frac{Z+2.414B}{Z-0.414B}\right)
\]
The enthalpy departure of the a fluid is given by,
\[ H-H^{*}=RT(Z-1)+\frac{T\frac{da}{dT}-a}{2\sqrt{2}b}\ln\left(\frac{Z+2.414B}{Z-0.414B}\right)
\]
Henry's Law:
The mole fraction or partial pressure of ith component, $p_i$ is proportional to the mole fraction of ith component in the liquid phase, $x_i$,
\[ p_{i}=H_{i}x_{i}
\]
For
ideal gas,
\[ p_{i}=y_{i}P
\]
Thus, a linear equilibrium relationship is obtained,
\[ \frac{y_{i}}{x_{i}}=\frac{H_{i}}{P}
\]
For pure gas adsorption at pressure P, the number of moles adsorbed on the surface, n, is given by Henry's law for adsorption,
\[ n=kP
\]
$H_i$ is Henry constant; $P$ is total pressure; $y_i$ is mole fraction of ith component in vapor phase.
Newton's Law of Viscosity:
The shear stress is proportional to shear rate,
\[ \tau_{yx}=-\mu\frac{dv_{x}}{dy}
\]
All the gases and liquids and solutions of low-molecular-weight compounds exhibit Newtonian behavior. Non-Newtonian behaviors are shown below,

Some dimensionless numbers, such as Prandtl and Schmidt numbers can be obtained for empirical correlations for heat and mass transfer.
Fourier's Law:
Heat transfer rate of conduction due to temperature difference is written as,
\[ \dot{q}=kA\frac{(T_{2}-T_{1})}{\triangle z}=-kA\frac{dT}{dz}
\]
The differentiation form may be used if heat transfer area is a variable,
\[ \frac{d}{dz}\left(A\frac{dT}{dz}\right)=0
\]
Convective heat transfer at low temperature by Newton's law of cooling,
\[ \dot{q} = hA(T_2 - T_1)
\]
$k$ is the thermal conductivity; and $h$ is the heat transfer coefficient.
Fick's First Law:
For steady-state diffusion without convection, Fick's first law applies. The molar flux is described as,
\[ J_{A}=-CD_{AB}\nabla x_{A}\underrightarrow{C\,is\,constant}-D_{AB}\nabla C_{A}\underrightarrow{1-D}-D_{AB}A\left(\frac{dC_{A}}{dz}\right)
\]
where, $D_{AB}$ is the diffusion coefficient of A in B; $J_A$ is the molar flux of A; $C_A$ is the molar concentration of A; $x_A$ is the mole fraction of A.
Or the Fick's law gives the diffusive flux relative to the average velocity of fluid mixture. In absence of convection, the diffusive flux and the total flux are the same. For diffusion relative to fixed/stationary coordinates, the molar flux becomes,
\[ N_{A}=J_{A}+vC_{A}=-C_{A}D_{AB}\nabla x_{A}+v^* C_{A}
\]
where, average velocity, $v^*$ is $x_A v_A + x_B*v_B$.
\[ N = N_A + N_B = Cv^* = C_A v_A + C_B v_B
\]
Since the mass transfer results in some amount of convection (diffusional flux), the first law is valid only for dilute solutions or in cases of diffusion in solids. In concentrated solutions, the diffusion coefficient depends on the concentration.
Fick's Second Law:
It describes how diffusion causes the concentration to change with time.
\[ \frac{\partial C}{\partial t}=D_{AB}\frac{\partial C^{2}}{\partial z^{2}}
\]
Film Model:
The assumptions for film theory:
- The entire variation of concentration or temperature takes place within a thin fluid film adjacent to the interface. While, in the bulk fluid, the concentration or temperature is almost uniform.
- Within the film, the mass or heat transfer takes place by steady-state 1-D diffusion or conduction, respectively.
The mass transfer flux for species A is written in terms of diffusion coefficient, concentrations at the two faces of the fictitious film and film thickness,
\[ N_{A}=D_{AB}\frac{\left(C_{A2}-C_{A1}\right)}{\delta_{m}}
\]
Note that $C_{A2}$ is same as the concentration in bulk flow.
But in terms of mass transfer rate, $k$, which is measurable by experiment,
\[ N_{A}=k(C_{A2}-C_{A1})
\]
Differently, the mass transfer coefficient depends on the hydrodynamics, i.e. the fluid flow field in the bulk fluid.
Thus, the film thickness can be obtained,
\[ \delta_{m}=\frac{D_{AB}}{k}
\]
Two-Film Theory:
For two-phase flow, the mass transfer across the interface can be simplified:
- The resistance to mass transfer lies in two different fictitious films adjacent to the interface on both of its sides.
- The concentration of the chemical species in both phase at the interface is in equilibrium (insignificant mass transfer across the interface).
The total mass transfer coefficients $K_g$ and $K_l$ can be expressed in term of the individual mass transfer coefficient (when Henry's law applies):
\[ \frac{1}{K_{g}}=\frac{1}{k_{g}}+\frac{H}{k_{l}}
\]
\[ \frac{1}{K_{l}}=\frac{1}{k_{l}}+\frac{1}{Hk_{g}}
\]
If the mass transfer in the liquid phase is controlling, then kg>>kl and hence $K_l=k_l$. Vice versa.
Similarly, this also applies to heat transfer, where constant temperatures are canceled.
\[ \frac{1}{U}=\frac{1}{h_{1}}+\frac{1}{h_{2}}
\]
Arrhenius' Law:
The chemical reaction rate can be the product of two terms, one depends on concentration (associated with pressure terms) and one depends on temperature only.
\[ -r_{A}=f\left(C_{A},C_{B},...\right)k^{'}(T)
\]
The temperature term can be written as follows, Arrhenius' Law,
\[ k^{'}(T)=k_{0}^{'}e^{-E/RT}
\]
$k^{'}$ is the kinetic rate expression; $k_0^{'}$ is the constant, E is the activation energy of the reaction.
Adsorption Isotherms:
The equilibrium relationship relating $q$ (the mount adsorbed on the solid) and $C_A$ (the concentration of the adsorbate in the fluid) is known as Isotherm. Sometimes, isotherms are given in terms of $K = K_a / K_d$ (rates of adsorption and desorption) and $\theta = q / q_0$ ($q_0$ is the adsorption capacity of adsorbent). Commonly used adsorption isotherms are,
- Langmuir (1918), no interaction between the adsorbed molecules
\[ p=\frac{1}{K}\left(\frac{\theta}{1-\theta}\right)
\]
- Brunauer-Emmett-Teller (1938), $p_{r}=p/p_{s}$, $q_m$ = constant
\[ \frac{q}{q_{m}}=\frac{K_{B}p_{r}}{(1-p_{r})(1-p_{r}+K_{B}p_{r})}
\]
- Freundlich (1926) Empirical
\[ q=k_{F}C^{1/n_{F}}
\]
- Raddke and Prausnitz (1972) combines Freundlich isotherms and Henry's law
\[ q=\frac{1}{\left(\frac{1}{K_{Hp}}+\frac{1}{k_{F}p^{1/n_{F}}}\right)}
\]
References:
Verma, Ashok Kumar. 2014. Process Modelling and Simulation in Chemical, Biochemical and Environmental Engineering. CRC Press.
http://en.wikipedia.org/wiki/Equation_of_state
http://kshmakov.org/fluid/note/3/